Click on the animation below for a full overview of the FLAURA trial
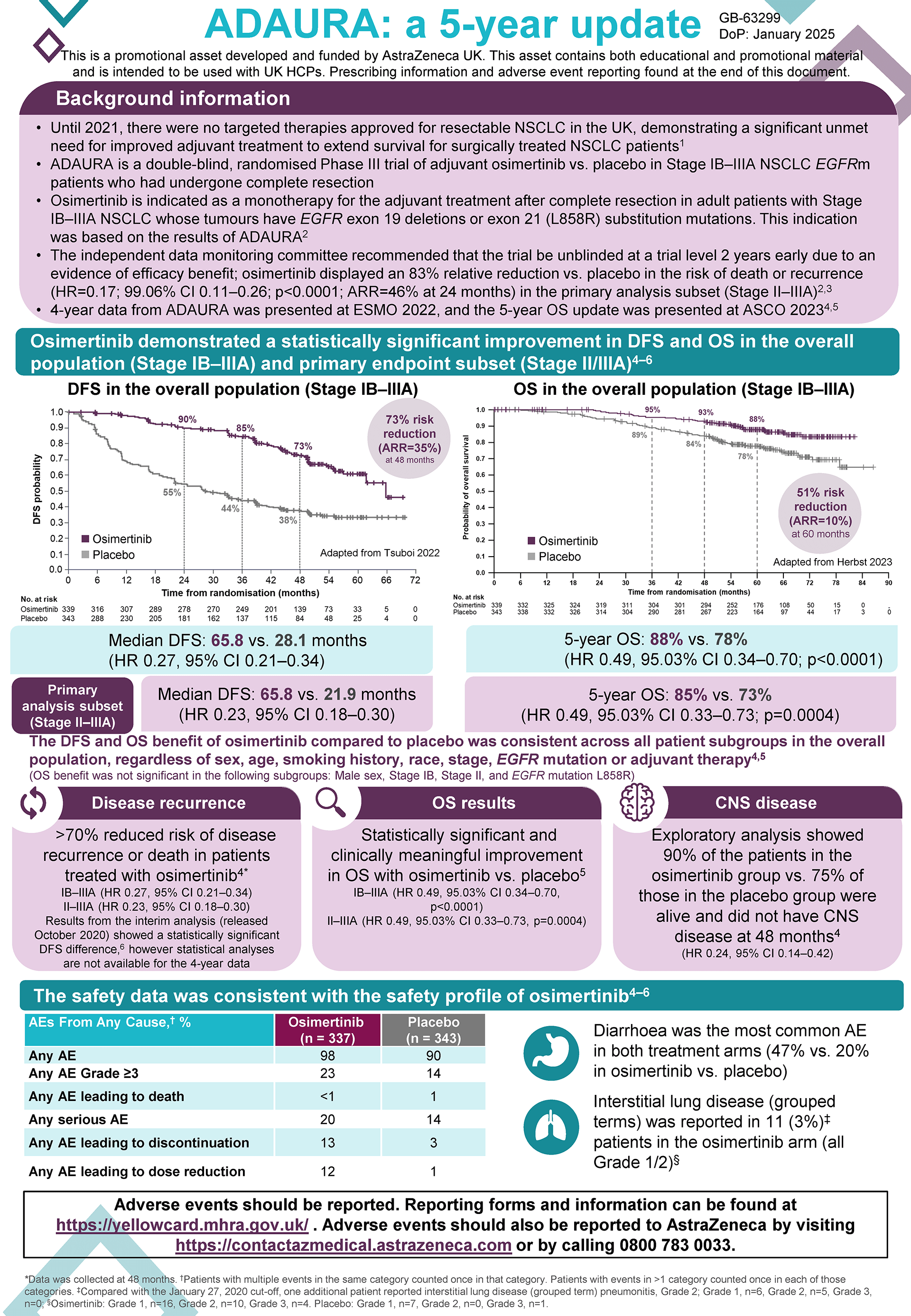
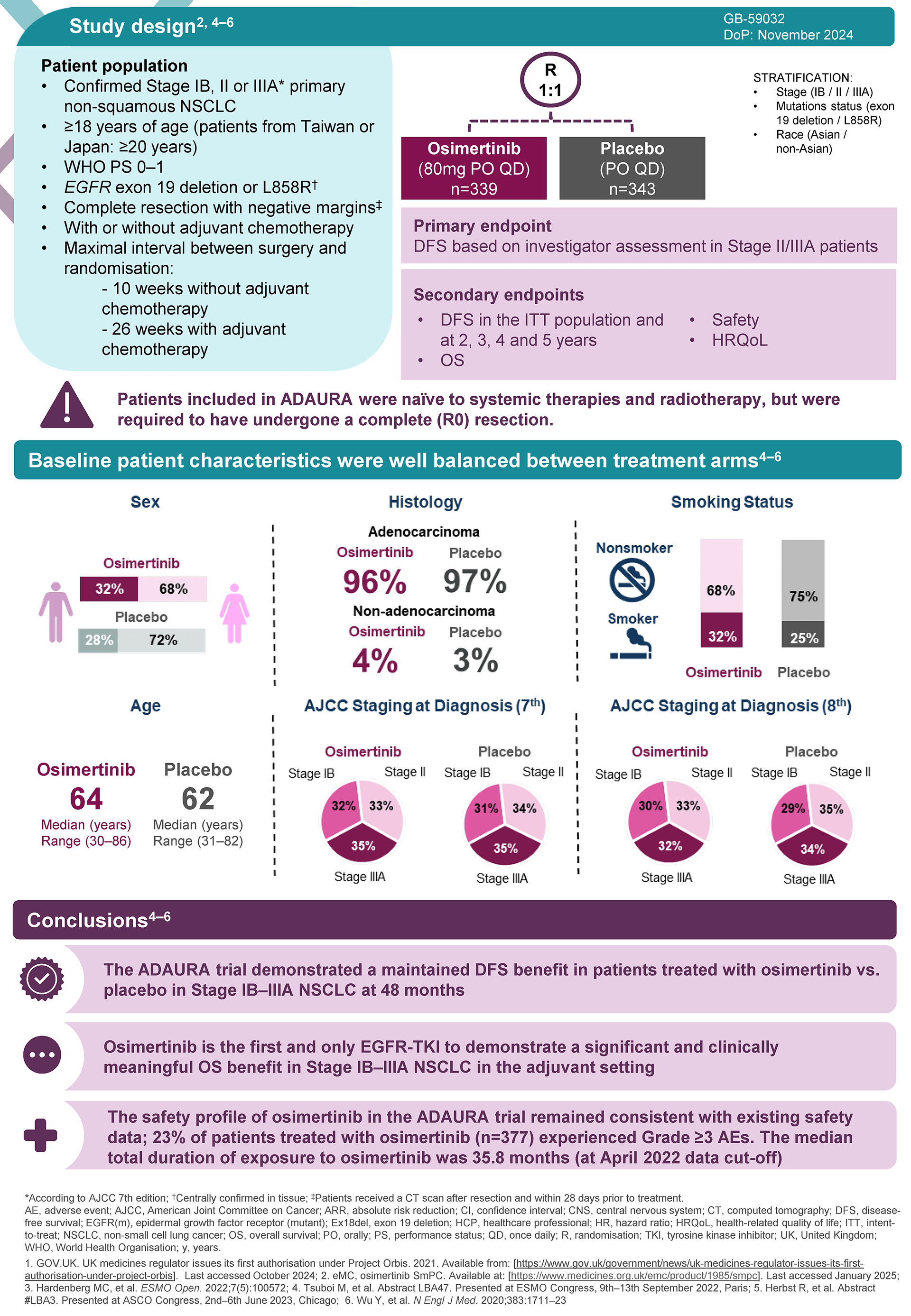
Background
The FLAURA trial was a double-blind, phase 3 trial involving patients with previously untreated advanced NSCLC (non-small cell lung cancer) with EGFR mutations that compared the efficacy and safety profiles of osimertinib with that of two other EGFR-TKIs, gefitinib or erlotinib.
Conclusion
Among patients with previously untreated advanced NSCLC with an EGFR mutation, those who received osimertinib (n=279) had longer overall survival (median OS 38.6 months), than those who received a comparator EGFR-TKI (n=277; median OS 31.8 months; p=0.046).1 The safety profile for osimertinib was similar to that of the comparator EGFR-TKIs, despite a longer duration of exposure in the osimertinib group.
Overall survival was a secondary endpoint. The primary endpoint was PFS; the median PFS was 18.9 months with osimertinib vs 10.2 months with comparator EGFR-TKI (p<0.001).2
References
- Ramalingam et al. N Engl J Med 2020; 382:41-50
- Soria et al. N Engl J Med 2018;378:113-125